Abstract
Background: Neihulizumab represents a novel immune checkpoint agonistic antibody that binds to human CD162 (PSGL-1), leading to downregulation of activated T-cells. Results of early-phase trials of neihulizumab have suggested a benefit in patients with auto-immune conditions, including psoriasis, psoriatic arthritis and ulcerative colitis. Here, we report preliminary results of a Phase I study investigating the use of neihulizumab for SR-aGVHD or TR-aGVHD (ClinicalTrials.gov Identifier: NCT03327857).
Aims: The aims of this study were to establish the pharmacokinetics, receptor occupancy (RO), safety and efficacy of neihulizumab in patients with SR-aGVHD or TR-aGVHD.
Methods: SR-aGVHD was defined as aGVHD that (1) progressed after 3 days of treatment with methylprednisolone (MP) 2 mg/kg/day equivalent, or (2) persisted after 7 days of treatment with MP 2 mg/kg/day equivalent, or (3) progressed to involve a new organ after treatment with MP 1 mg/kg/day equivalent for skin and upper gastrointestinal aGVHD, or (4) recurred during or after steroid taper. TR-aGVHD was defined as having received one prior systemic treatment for aGVHD in addition to corticosteroid. Participants with demonstrable skin SR-aGVHD were enrolled and received a single dose of neihulizumab in Cohort 1 at either 3 mg/kg (Dose Level 1 [DL 1]) or 6 mg/kg (Dose Level 2 [DL 2]). With the completion of Cohort 1 and without observation of dose limiting toxicities, participants with any grade SR-aGVHD were enrolled for multiple dose treatment of neihulizumab (Cohort 2) with first dose at 6 mg/kg followed by weekly doses of 4 mg/kg for 3 weeks (6-4-4-4 regimen).
Results: A total of 37 participants were enrolled with 13 participants in Cohort 1 and 24 participants in Cohort 2.
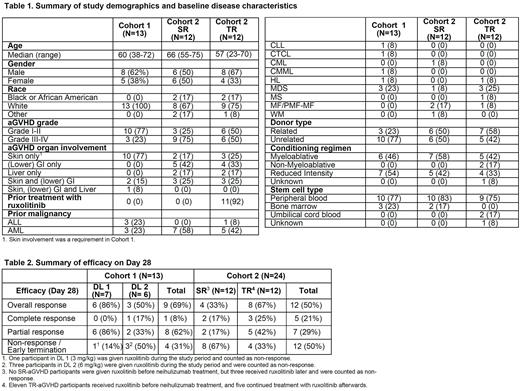
In Cohort 1, all 13 participants had SR-aGVHD with erythematous manifestation of cutaneous aGVHD. In Cohort 2, 12 participants had SR-aGVHD and 12 had TR-aGVHD. Notably, 11 TR-aGVHD participants received ruxolitinib prior to neihulizumab treatment. A summary of study demographics and baseline disease characteristics is shown in Table 1.
In Cohort 1, at 4 hours after the end of the neihulizumab infusion, the median RO was 91% for both DL 1 and DL 2. At Day 8, median RO decreased to 69% (range 23-80%) in DL 1 but was maintained at 91% (range 90-102%) in DL 2. In Cohort 1, most frequently observed Grade 3-4 adverse events (AE, at least 5%) were lymphocyte count decreased, platelet count decreased, hyponatremia, hyperglycemia and white blood cell decreased. Day 28 overall response rate (ORR) was 86% in DL 1 and 50% in DL 2, including 1 complete response (CR). Details are described in Table 2.
In Cohort 2, 13 participants (54%) received all four doses of neihulizumab and reached the Day 28 aGVHD evaluation, 3 (13%) received three doses, and 8 received one or two doses. In this 6-4-4-4 dosing regimen, at 1 hour after the end of the first dose of neihulizumab infusion (6 mg/kg), the median RO was 93%. For subsequent weekly infusions (4 mg/kg) for three weeks, the median RO was maintained above 80% (range 84-93%) until Day 35. Day 28 ORR was 33% among SR-aGVHD participants, including 17% CR, and 67% among TR-aGVHD participants, including 25% CR (Table 2). The most frequently observed Grade 3-4 AEs (at least 5%) were anemia, platelet count decreased, hypocalcemia, lymphocyte count decreased, hypokalemia, hypoxia, ileus, sepsis and white blood cell decreased. Among the 24 participants in Cohort 2, 5 (42%) SR-aGVHD and 4 (33%) TR-aGVHD participants died during the study period (Day 180). The median survival was 122.5 and 124 days (by 26th July 2022) for SR-aGVHD and TR-aGVHD, respectively.
Summary/Conclusion: Neihulizumab appeared to be well-tolerated in participants with SR-aGVHD or TR-aGVHD. With the current 6-4-4-4 dosing regimen (6 mg/kg followed by 4 mg/kg weekly doses for 3 weeks), RO was maintained at least 80% throughout most of the treatment period. The Day 28 ORR was 69% in Cohort 1 and 50% in Cohort 2. The higher ORR in Cohort 1 is likely due to the participants with predominantly steroid refractory skin aGVHD. Of note, the ORR in participants with TR-aGVHD, of which 11 of 12 were receiving combination ruxolitinib therapy, appeared to be higher than in those with SR-aGVHD (67% vs 33%). These promising results support further investigation of neihulizumab as an agent in ruxolitinib-refractory SR-GVHD.
Disclosures
Abedin:AbbVie: Honoraria; Incyte: Research Funding; Amgen: Honoraria; Stemline: Honoraria; AltruBio Inc.: Research Funding; Helsinn Healthcare: Research Funding; Pfizer: Research Funding; Actinium Pharmaceuticals: Research Funding. Hamadani:Takeda: Research Funding; Incyte Corporation: Consultancy; Medical University of Wisconsin: Current Employment; Omeros: Consultancy; SeaGen: Consultancy; Genmab: Consultancy; MorphoSys: Consultancy; Gamida Cell: Consultancy; Abbvie: Consultancy; Kite: Consultancy; ADC Therapeutics: Consultancy, Research Funding, Speakers Bureau; Kadmon: Consultancy; Legend Biotech: Consultancy; Novartis: Consultancy; Sanofi Genzyme: Speakers Bureau; Spectrum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; AstraZeneca: Speakers Bureau; BioGene: Speakers Bureau. Holtan:Vitrac Therapeutics: Research Funding; Incyte: Research Funding; Ossium: Consultancy; Generon: Consultancy; CSL Behring: Other: Clinical trial adjudication. Schiller:AstraZeneca: Honoraria; Medimmune: Research Funding; Regimmune: Research Funding; Samus: Research Funding; Jazz: Consultancy; Constellation: Research Funding; Cyclacel: Research Funding; Stemline: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Gilead: Research Funding; Cellerant: Research Funding; Novartis: Honoraria, Other: Speaker fees, Research Funding; Arog: Research Funding; Gamida: Research Funding; Sellas: Research Funding; Forma: Research Funding; Genentech-Roche: Research Funding; Stemline: Speakers Bureau; Millennium: Research Funding; AVM Biopharma: Research Funding; Amgen: Current equity holder in publicly-traded company, Honoraria; AltruBio: Research Funding; PreCOG LLC: Research Funding; Geron: Research Funding; Pfizer: Research Funding; Actuate: Research Funding; Deciphera: Research Funding; Daiichi-Sankyo: Research Funding; Deltafly: Research Funding; FujiFilm: Research Funding; Glycomimetics: Research Funding; Karyopharm: Research Funding, Speakers Bureau; Johnson & Johnson: Current equity holder in publicly-traded company; Mateon: Research Funding; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; CTI: Research Funding; Ono Pharma: Honoraria; Janssen: Research Funding; Kite, a Gilead Company: Research Funding, Speakers Bureau; Cellectis: Research Funding; Trovagen: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; Astellas: Research Funding, Speakers Bureau; Agios: Consultancy, Honoraria; Actinium: Research Funding; AbbVie: Research Funding, Speakers Bureau; Onconova: Research Funding; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding. Waller:Orca Bio: Research Funding; Verastem Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kosuri:Seres Therapeutics: Research Funding. Abhyankar:Therakos: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Research Funding, Speakers Bureau. Hall:AltruBio: Current Employment, Current holder of stock options in a privately-held company. Martin:AltruBio (formerly AbGenomics): Research Funding; Enlivex Pharmaceuticals: Consultancy; Mesoblast: Consultancy; Pfizer: Other: Data and Safety Monitoring Board; Procter and Gamble: Current equity holder in publicly-traded company; Rigel: Consultancy; Talaris: Consultancy; Xenikos: Research Funding; Janssen Pharmaceuticals: Honoraria; Mallinckrodt: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal